 |
Functional group specific derivatization reactions used to quantify hydroxyl, carbonyl and carboxylic acid group concentrations on carbonaceous surfaces |
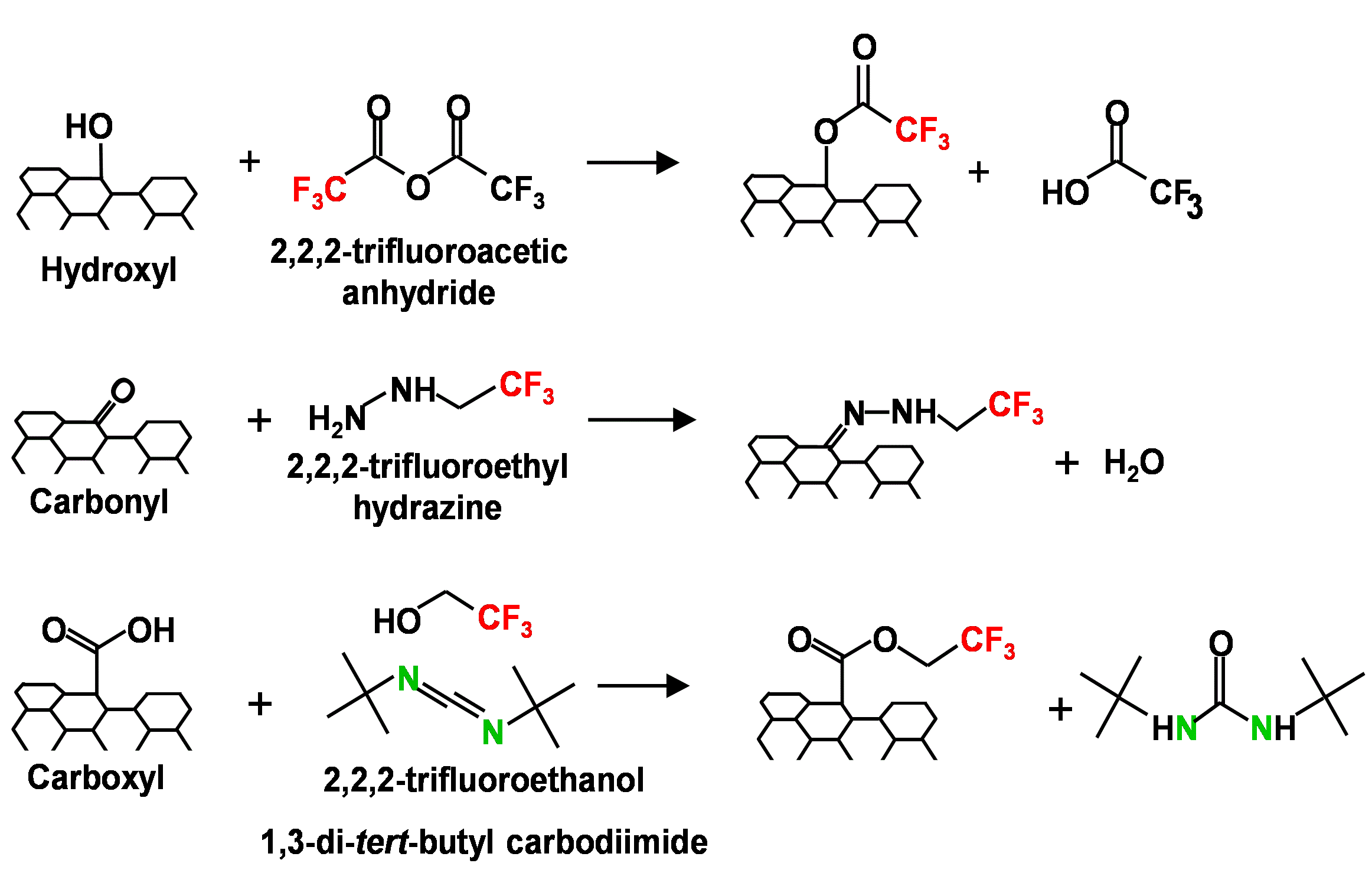
Carbonaceous materials such as carbon nanotubes (CNTs), fullerenes and activated carbons often contain hydrophilic oxygen-containing functional groups on their surfaces. These surface oxides are introduced either deliberately to improve dispersion or sorption properties, or unintentionally after the carbonaceous material has been released into the environment and exposed to oxidizing agents (e.g. ozone or hydroxyl radicals). The influence of these surface oxides depends on both the total oxygen concentration and the distribution of functional groups. Although the total oxygen concentration on the surface can easily be quantified by X-ray Photoelectron Spectroscopy (XPS) an unambiguous determination of the distribution of surface oxides (e.g. hydroxyl vs. carboxyl group) is far more challenging. To address this issue we have developed a number of functional group specific derivatizing reactions (Figure right) that can quantify the concentration of hydroxyl, carbonyl and carboxylic acid groups on carbonaceous surfaces by measuring fluorine uptake with XPS. The Fairbrother group has used this information to develop relationships between CNT surface chemistry and environmentally relevant properties in aqueous environments, such as particle stability and sorption properties. We have also used these reactions to assist other researchers in studies involving carbon surfaces; most recently Professor David Cwiertny at the University of Iowa and Professor Brian Chaplin at Villanova.